Abstract
Post-traumatic stress disorder (PTSD) leads to enhanced alcohol drinking and development of alcohol use disorder (AUD). Identifying shared neural mechanisms might help discover new therapies for PTSD/AUD. Here, we employed a rat model of comorbid PTSD/AUD to evaluate compounds that inhibit FK506-binding protein 51 (FKBP5), a co-chaperone modulator of glucocorticoid receptors implicated in stress-related disorders. Male and female rats received a familiar avoidance-based shock stress followed by voluntary alcohol drinking. We then assessed trauma-related behaviors through sleep bout cycles, hyperarousal, fear overgeneralization, and irritability. To evaluate the role of stress and alcohol history on the sensitivity to FKBP5 inhibitors, in two separate studies, we administered two FKBP5 inhibitors, benztropine (Study 1) or SAFit2 (Study 2). FKBP5 inhibitors were administered on the last alcohol drinking session and prior to each trauma-related behavioral assessment. We also measured plasma corticosterone to assess the actions of FKBP5 inhibitors after familiar shock stress and alcohol drinking. Benztropine reduced alcohol preference in stressed males and females, while aggressive bouts were reduced in benztropine-treated stressed females. During hyperarousal, benztropine reduced several startle response outcomes across stressed males and females. Corticosterone was reduced in benztropine-treated stressed males. The selective FKBP5 inhibitor, SAFit2, reduced alcohol drinking in stressed males but not females, with no differences in irritability. Importantly, SAFit2 decreased fear overgeneralization in stressed males and females. SAFit2 also reduced corticosterone across stressed males and females. Neither FKBP5 inhibitor changed sleep bout structure. These findings indicate that FKBP5 inhibitors modulate stress-related alcohol drinking and partially modulate trauma-related behaviors. This work supports the hypothesis that targeting FKBP5 may alleviate PTSD/AUD comorbidity.
Similar content being viewed by others
Introduction
Excessive alcohol use is a major economic and public health problem that is aggravated in individuals with post-traumatic stress disorder (PTSD) [1,2,3]. Individuals with PTSD display the greatest prevalence rates for alcohol use disorder (AUD) [1] and show poorer health outcomes [4] and higher rates of suicidality [5]. These negative consequences are magnified among military Veterans with comorbid PTSD/AUD including higher rates of AUD [6], 3-fold higher suicidal ideations [7], and higher aggression indices [8]. Thus, there is a need to understand the underlying mechanisms to assist in developing therapeutic strategies to alleviate trauma-related alcohol drinking.
Recent advances have suggested that FK506-binding protein 51 (FKBP5), a glucocorticoid receptor (GR) co-chaperone, as a promising novel therapeutic target [9, 10] to counteract over-active shared stress systems associated with alcohol use [9,10,11,12]. Epidemiological studies have also suggested that FKBP5 gene variants predict greater alcohol use [13]. A function of FKBP5 is to decrease the ligand binding affinity of GR, in turn delaying nuclear translocation and decreasing GR transcriptional activity [14, 15]. Enhanced FKBP5 activity is associated with increased corticosterone and blunted negative feedback inhibition of the stress axis [16], providing a basis to study FKBP5 inhibition as a potential avenue to increase negative feedback of stress systems and reduce comorbid stress disorders and alcohol use. In rodent models, stress exposure also increases FKBP5 expression in numerous brain regions associated with drug use [17]. Mice with a history of maternal separation stress and alcohol exposure also showed higher levels of Fkbp5 gene expression in cortical regions [13]. Thus, here, we examined the role of stress and alcohol history on the sensitivity to compounds that inhibit FKBP5 in our adapted model of comorbid PTSD/AUD [18].
Inhibitory avoidance-based shock stress models have been widely used to study stress-mediated drinking and anxiety across species of rodents [19,20,21,22]. We recently developed a familiar avoidance-based shock stress model of PTSD that elicits heightened alcohol drinking, fear overgeneralization, and irritability after rats experience “2-hit” traumatic shocks in a familiar environment [18, 23]. This translationally relevant model utilizes Pavlovian and instrumental conditioning of inhibitory avoidance that encompasses Pavlovian non-associative sensitization to fear, and instrumental aspects related to negative reinforcement [24]. The changes in alcohol drinking and anxiety-like phenotypes also translationally mimic psychogenic symptoms of comorbid PTSD/AUD. In addition, this model encompasses sex differences whereby stressed females display increased hyperarousal and decreased sleep maintenance during abstinence from alcohol when compared to stressed males [18].
We utilized this model of comorbid PTSD/AUD in a familiar contextual environment in male and female rats [18] and tested two FKBP5 inhibitors in two separate studies: benztropine (Study 1), an FDA-approved drug (Cogentin®) with repurposing potential that disrupts the association of FKBP5 with the glucocorticoid receptor (GR)-heat shock protein 90 complexes [25]; and SAFit2 (Study 2), a target-selective FKBP5 inhibitor [26]. Changes in alcohol drinking, hyperarousal, diurnal sleep patterns, fear overgeneralization, and irritability-like behavior were measured following administration of benztropine or SAFit2 during abstinence from alcohol. To better understand effects of treatment on peripheral stress indices, we analyzed plasma corticosterone. Overall, we hypothesized that the FKBP5 inhibitors would reduce comorbid phenotypes of PTSD/AUD, and that these effects would demonstrate important sex differences in trauma-related behavioral responses and potentially in corticosterone.
Methods and materials
The Supplementary material includes detailed descriptions of all experimental procedures.
Animals
A total of 176 adult male (~300 g) and female (~200 g) Wistar rats were purchased from Charles River Laboratories. All rats were pair-housed separated by a perforated clear Plexiglas® divider and had access to food and water ad libitum. All experimental procedures were approved by The Scripps Research Institute Institutional Animal Care and Use Committee (Protocol #: 19-00024 and ACURO #:5-312-216591). All procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition).
Experimental design overview
This report consists of two studies conducted in separate cohorts of unstressed and stressed rats (n = 8–10 per group). Study 1 examined the effects of benztropine (Cogentin®), a broad-acting and FDA-approved drug with FKBP5 inhibiting properties, and Study 2 examined a selective FKBP5 inhibitor, SAFit2, on alcohol drinking, diurnal sleep cycles, hyperarousal, fear overgeneralization, and irritability following familiar inhibitory avoidance shock stress in male and female rats. Unstressed control animals underwent similar procedures without the presence of the shock. Administration of FKBP5 inhibitors occurred on the last day of alcohol drinking and again prior to each PTSD-like behavioral test that was assessed 1 week apart. Blood plasma was collected to assess the acute effects of FKBP5 inhibitors on circulating corticosterone levels on the last day of alcohol drinking. See Fig. 1 for experimental design overview.
To evaluate the role of stress and alcohol history on sensitivity to FKBP5 inhibitors, we administered benztropine (Study 1), a broad-acting FKBP5 inhibitor, or SAFit2 (Study 2), a target-selective FKBP5 inhibitor. Rats first received an inhibitory avoidance-based “2-hit” familiar shock stress. Two weeks later, all male (n = 8–10 per group) and female (n = 8–10 per group) rats then received 2-bottle choice (2BC) alcohol drinking for 4 weeks (20%; 2 h; Monday, Wednesday, Friday). On the last day of alcohol access, rats received an acute administration of vehicle or benztropine (0, 5, 10 mg/kg; IP) or SAFit2 (0, 10, 20 mg/kg; IP). Plasma corticosterone was also assessed at the end of the alcohol test day. The rats then received a 1-week break period and then were tested on trauma-related behaviors which include sleep bouts cycles using the comprehensive lab animal monitoring system (CLAMS), acoustic startle, fear context overgeneralization, and bottle-brush irritability. Benztropine or SAFit2 were re-administered on each trauma-related behavioral test day. Each behavioral test was separated 1-week apart. Unstressed controls underwent similar procedures without the presence of the familiar shock stress and were conducted as separate cohorts.
Drugs
Benztropine was administered 2 h prior to each test [27], and the doses selected were 5 and 10 mg/kg based on prior work [27]. SAFit2 was administered 16 h prior to each test [28], and the doses selected were 10 and 20 mg/kg based on prior literature [29, 30]. The different pretreatment intervals (2 vs 16 h) were based on previous studies with these compounds [26,27,28, 31,32,33].
Familiar shock stress
The “2-hit” foot shock stress procedures were conducted using a familiar context as previously reported [18]. The foot shock stress (3.0 mA for 2 s) occurred on two single shock instances in the same contextual environment (familiar) 48 h apart. Latency to cross (s) to the dark compartment was recorded.
Voluntary alcohol regimen
Two weeks after the first foot shock, rats received an initial 48 h acclimation to the alcohol (20% v/v) in addition to the water bottle followed by intermittent (Monday, Wednesday, Friday), 2-bottle choice (2BC) limited access (2 h) for 4 weeks in their home cage as previously described [18]. Blood alcohol levels were measured on the last 2BC alcohol session before rats entered their abstinence period. To validate measures, intake of rats that still showed detectable alcohol levels by the end of the 2 h session were compared to those that no longer showed detectable levels (see Supplementary Fig. S6).
Comprehensive Lab Animal Monitoring System (CLAMS)
Rats were placed in a OXYmax-CLAMS units (Columbus Instruments) to assess their sleep maintenance during their inactive phase (lights on). Number of sleep bouts, average bout duration (min), max bout duration (min), total sleep (min), and total sleep (%) were recorded as previously reported [34].
Acoustic startle
Acoustic startle responses were recorded on a SR-Lab system (SR-LAB) as previously reported [18, 34]. Rats underwent a 5-min acclimation period in the startle chamber before the beginning of 75 pseudorandomized trials across a 30-min testing window.
Fear overgeneralization
To measure fear-related context memory, all rats were tested in a fear overgeneralization paradigm in a modified inhibitory avoidance box and were given a maximum of 10 min to enter the dark compartment as previously described [18, 23]. Latency to cross (s) into the dark compartment was recorded, and animals that never crossed were censored at 10 min (600 s).
Bottle brush irritability
Behaviors assessed were aggressive behaviors, which included biting, boxing, following, and mounting, as well as defensive behaviors, including startling, digging, freezing, climbing cage walls, vocalizing and attempting to escape and general explorative behaviors such as grooming, rearing, and exploring as described in ref. [18].
Corticosterone analysis
Plasma samples were analyzed using commercially available MilliPlex® kits (Millipore Sigma) specific for corticosterone as previously reported [18]. Blood plasma collection was conducted for all rats immediately after drinking (4 h into their dark cycle) on the same day of the treatment with FKBP5 inhibitors. The samples were analyzed on a MAGPIX® system using xPONENT® software. The intra-assay (%) CVs were <10%.
Statistical analysis
Separate generalized linear models (ANOVA or rank regression) were used to analyze outcome data from each experiment and sex in Study 1 (Benztropine) and Study 2 (SAFit2). Model 1 analysis only involved stressed groups and included drug (Benztropine: 0, 5 or 10 mg/kg and SAFit2: 0, 10 or 20 mg/kg) as between-subject factors. Model 2 included stress condition (unstressed versus stressed), drug (Benztropine: 0 or 10 mg/kg and SAFit2: 0 or 20 mg/kg), and the interaction of stress and drug as between-subject factors. Corticosterone data were analyzed in a similar manner. A sub-analysis derived from Model 2 was used to compare vehicle-treated unstressed versus stress groups in both Study 1 and Study 2. Behavioral outcome data were performed using SAS version 9.4 (SAS Institute Inc.). Blood plasma data were analyzed on GraphPad Prism Version 8. Adjustments for multiple comparisons were conducted by using the false discovery rate (FDR) correction method. Full linear model results tables are illustrated in Supplementary Tables S1–S7. We report significance values throughout the results and figure legends. All graphs were generated using GraphPad Prism version 8.
Results
“2-hit” shock stress in a familiar environment increased alcohol intake and some trauma-related behaviors
We compared vehicle-treated unstressed versus shock-stressed rats (from Model 2 analysis) and found stress phenotypes generally consistent in direction and magnitude with prior work using the familiar “2-hit” model described in Steinman et al. (2021). To allow comparison to prior work and across cohorts, we report in Supplementary Tables S1 and S2 the mean differences for the observed effect of stress in vehicle-treated controls.
FKBP5 inhibitors reduce alcohol drinking after familiar shock stress history
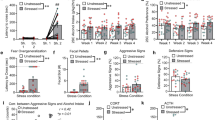
We measured whether benztropine (Study 1) or SAFit2 (Study 2) decreased 2BC alcohol drinking following familiar shock stress history. In Study 1, our Model 1 analysis revealed that benztropine significantly reduced alcohol preference in shock-stressed males at the 5 mg/kg dose versus vehicle-treated controls (p < 0.05; Fig. 2b and Supplementary Table S4) but not alcohol intake (p > 0.05; Fig. 2a and Supplementary Table S4). There were no differences observed in our Model 2 analysis across male groups for alcohol intake or preference (p’s > 0.05; Fig. 2c, d and Supplementary Table S5). Our Model 1 analysis in shock-stressed females revealed that benztropine also reduced alcohol preference at the 5 and 10 mg/kg dose versus vehicle-treated controls (p < 0.05; Fig. 2f and Supplementary Table S4) but not alcohol intake (p > 0.05; Fig. 2e and Supplementary Table S4). Our Model 2 analysis revealed that benztropine generally reduced alcohol preference in females (p < 0.05; Fig. 2h and Supplementary Table S5), but not alcohol intake (p > 0.05; Fig. 2g and Supplementary Table S5).
The top panels (a–h) reflect 2BC alcohol drinking following benztropine administration (Study 1: n = 8–10 per group; 0, 5, or 10 mg/kg) and the bottom panels (i–p) reflect alcohol drinking following SAFit2 administration (Study 2: n = 8–10 per group; 0, 10, or 20 mg/kg). Data are expressed as mean (±SEM) for alcohol intake (g/kg/2 h) or preference (%). The asterisks (*) denote a significant difference from vehicle-treated rats and the number sign (#) reflects a difference from unstressed control, *p < 0.05, **p < 0.01, ***p < 0.001 or #p < 0.05.
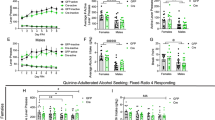
In Study 2, our Model 1 analysis in shock-stressed males revealed that SAFit2 reduced alcohol intake and preference at the 10 and 20 mg/kg dose versus vehicle-treated controls (p’s < 0.05; Fig. 2i, j and Supplementary Table S6). Similarly, our Model 2 analysis also revealed that SAFit2 reduced alcohol intake in shock-stressed males at the 20 mg/kg dose versus vehicle-treated controls (p < 0.05; Fig. 2k and Supplementary Table S7). Across female groups, Model 1 and Model 2 identified no differences produced by SAFit2 for alcohol intake or preference (p’s > 0.05; Fig. 2m–p and Supplementary Tables S6 and S7).
FKBP5 inhibitors reduce some trauma-related behaviors after familiar shock stress and alcohol drinking history
FKBP5 inhibitors do not alter sleep bout cycles
We measured the effects of FKBP5 inhibitors on sleep bout cycles after familiar shock stress and alcohol drinking using CLAMS. In Study 1, our Model 1 and Model 2 analysis revealed that benztropine did not produce significant differences in the longest sleep bout (min) across male and female groups (p’s > 0.05; Supplementary Fig. S1a–d and Supplementary Tables S4 and S5).
In Study 2, Model 1 and Model 2 analysis also revealed that SAFit2 did not produce significant differences in the longest sleep bout (min) across male and female groups (p’s > 0.05; Supplementary Fig. S1e–h and Supplementary Tables S6 and S7).
FKBP5 inhibitors reduce startle response outcomes after familiar shock stress and alcohol drinking history
One week after sleep bout cycles assessments, we measured whether the FKBP5 inhibitors reduced hyperarousal states after familiar shock stress and alcohol drinking. In Study 1, our Model 1 analysis in shock-stressed males revealed that benztropine reduced 120 dB startle responses (mV) during trial 1 and final block at the 10 mg/kg dose versus vehicle-treated controls (p’s < 0.05; Supplementary Fig. S2a, c and Supplementary Table S4) but not across trial 2–6 (p > 0.05; Supplementary Fig. S2b and Supplementary Table S4) and 80–105 dB series (p > 0.05; Supplementary Fig. S3a and Supplementary Table S4). Our Model 2 analysis also revealed that benztropine produced reductions in 120 dB startle responses (mV) in males during trial 1 and final block and across 80–120 dB startle responses versus vehicle-treated groups (p’s < 0.05; Supplementary Figs. S4a, c and S5a and Supplementary Table S5) but not across trial 2–6 (p > 0.05; Supplementary Fig. S4b and Supplementary Table S5). In shock-stressed females, our Model 1 analysis revealed that benztropine reduced 120 dB startle response during the final block phase and across 80–105 dB stimuli (p’s < 0.05; Supplementary Figs. S2g and S4b and Supplementary Table S5) at the 5 and 10 mg/kg dose versus vehicle-treated controls, but not across trial 1 or trials 2–6 (p’s > 0.05; Supplementary Fig. S2e, f and Supplementary Table S6). Model 2 analysis also revealed that females display general reductions in 120 dB startle responses during the final block phase and across 80–120 dB startle responses (p < 0.05; Supplementary Figs. S4g and S5b and Supplementary Table S5). There were no differences produced by benztropine for prepulse inhibition across male and female groups (p’s > 0.05; Supplementary Figs. S2d, h and S4d, h and Supplementary Table S5).
In Study 2, our Model 1 analysis revealed that SAFit2 reduced startle responses across 80–105 dB startle responses in shock-stressed males at the 20 mg/kg dose versus vehicle-treated controls (p < 0.05; Supplementary Fig. S3c and Supplementary Table S6). There were no differences in our Model 1 analysis produced by SAFit2 across trial 1, trial 2–6, and final block (p’s > 0.05; Supplementary Fig. S2i–l and Supplementary Table S6). Our Model 2 analysis revealed that SAFit2 produced general reductions during 120 dB trials 2–6 and across 80–120 dB startle responses at the 20 mg/kg dose versus vehicle-treated groups (p’s < 0.05; Supplementary Figs. S4j and S5c and Supplementary Table S7) but not across trial 1 and final block (p’s > 0.05; Supplementary Fig. S3i, k and Supplementary Table S7). Lastly, we also observed no significant differences in Model 1 and Model 2 analysis produced by SAFit2 in males and females during prepulse inhibition (p’s > 0.05; Supplementary Figs. S2p and S3p and Supplementary Tables S6 and S7).
The selective FKBP5 inhibitor, SAFit2, reduces fear overgeneralization after familiar shock stress and alcohol drinking history
These data are illustrated as violin plots and were analyzed using rank regression modeling to account for non-normal distribution of the data where many rats were censored at 600 s during testing. In Study 1, our Model 1 analysis revealed no significant differences in shock-stressed males following benztropine administration versus vehicle-treated controls (p > 0.05; Fig. 3a). However, Model 2 analysis revealed that benztropine administration generally increased the latency to cross (min) into the dark compartment of the apparatus in males at the 10 mg/kg dose versus vehicle-treated controls (p < 0.05; Fig. 3b). Furthermore, our Model 1 and Model 2 analysis in females revealed no differences produced by benztropine for latency to cross into the dark compartment (p > 0.05; Fig. 3c, d).
The top panels (a–d) reflect violin plots for fear overgeneralization in males and females following benztropine administration (Study 1: n = 8–10 per group; 0, 5 or 10 mg/kg) and the bottom panels (e–h) reflect fear overgeneralization in males and females following SAFit2 administration (Study 2: n = 8–10 per group; 0, 10, or 20 mg/kg). Data are expressed as violin plots that display median and quartiles for latency to cross (s) into the dark compartment of the fear overgeneralization apparatus. The asterisks (*) denote a significant difference from vehicle-treated rats and the number sign (#) reflects a difference from unstressed controls, *p < 0.05 or ##p < 0.05.
In Study 2, our Model 1 analysis in shock-stressed males revealed that SAFit2 reduced the latency to cross (min) into the dark compartment at the 20 mg/kg dose versus vehicle-treated controls (p < 0.05; Fig. 3e and Supplementary Table S6). There were no differences observed in our Model 2 analysis regarding SAFit2 effects on fear overgeneralization across male groups (p > 0.05; Fig. 3f and Supplementary Table S7). Furthermore, our Model 1 analysis in shock-stressed females revealed that SAFit2 reduced the latency to cross (min) into the dark compartment at the 10 mg/kg versus vehicle-treated controls (p < 0.05; Fig. 3e and Supplementary Table S6). Lastly, our Model 2 analysis revealed no differences produced by SAFit2 in females (p > 0.05; Fig. 3h).
Benztropine reduces aggressive bouts in females but not males
In Study 1, our Model 1 analysis in shocked stress males revealed no differences produced by benztropine across doses (p > 0.05; Fig. 4a and Supplementary Table S4). However, our Model 2 analysis revealed that benztropine generally reduced aggressive bouts in males at the 10 mg/kg dose versus vehicle-treated controls (p < 0.05; Fig. 4b and Supplementary Table S5). Furthermore, our Model 1 analysis in shock-stressed females revealed that benztropine reduced aggressive bouts at the 5 and 10 mg/kg dose versus vehicle-treated controls (p’s < 0.05; Fig. 4c and Supplementary Table S4). Our Model 2 analysis revealed similar marginal reductions in shock-stressed females in aggressive bouts at the 10 mg/kg dose versus vehicle-treated controls (p = 0.058; Fig. 4d and Supplementary Table S5).
The top panels (a–d) reflect aggressive bouts in the bottle brush test following benztropine administration (Study 1: n = 8–10 per group; 0, 5, or 10 mg/kg) and the bottom panels (e–h) reflect aggressive bouts following SAFit2 administration (Study 2: n = 8–10 per group; 0, 10, or 20 mg/kg). Data are expressed as mean (±SEM) for aggressive bouts. The asterisks (*) denote a significant difference from vehicle-treated control rats and the number sign (#) reflects a difference from unstressed controls, *p < 0.05, **p < 0.01 or #p < 0.05.
In Study 2, our Model 1 and Model 2 analysis revealed no significant differences by SAFit2 in aggressive bouts across male and female groups (ps > 0.05; Fig. 4e–h and Supplementary Tables S6 and S7).
FKBP5 inhibitors decrease corticosterone levels after familiar shock stress and alcohol drinking history
We assessed circulating levels of corticosterone from blood plasma collected at the end of the last 2BC session after administration of benztropine (Study 1) or SAFit2 (Study 2). In Study 1, our Model 1 and Model 2 analysis revealed that benztropine administration decreased corticosterone in stressed males at the 10 mg/kg dose (p’s < 0.05; Fig. 5a, b and Supplementary Table S3). In females, our Model 2 analysis revealed that benztropine generally increased corticosterone at the 10 mg/kg dose versus vehicle-treated groups (p < 0.05; Fig. 5d and Supplementary Table S3). Model 1 revealed that benztropine did not change corticosterone levels in stressed females (p > 0.05; Fig. 5c and Supplementary Table S3).
The top panels (a–d) reflect plasma corticosterone levels following benztropine administration (Study 1: n = 8–10 per group; 0, 5, or 10 mg/kg) and the bottom panels (e–h) corticosterone levels following SAFit2 administration (Study 2: n = 8–10 per group; 0, 10, or 20 mg/kg). Data are expressed as pg/mL (±SEM) for corticosterone. The asterisks (*) denote a significant difference from vehicle-treated controls and the number signs (#) denote a difference from unstress controls, *p < 0.05, **p < 0.01, *** p < 0.001 or, #p < 0.05, or ###p < 0.001.
In Study 2, our Model 1 and Model 2 analysis revealed that SAFit2 reduced corticosterone in stressed drinking males at the 10 and 20 mg/kg dose versus vehicle-treated groups (p’s < 0.05; Fig. 5e, f and Supplementary Table S3). Similarly, our Model 1 and Model 2 analysis revealed that SAFit2 also reduced corticosterone levels in stressed females at the 20 mg/kg dose versus vehicle-treated groups (p’s < 0.05; Fig. 5g, h and Supplementary Table S3). Lastly, we acknowledge differences in our corticosterone results between unstressed controls across Study 1 and Study 2. This discrepancy may relate to differences in our pretreatment injection intervals, cohort variability, and prior benztropine or SAFit2 treatment effects.
Discussion
This report studied the effects of two FKBP5 inhibitors in an adapted model of comorbid PTSD/AUD [18]. Our model elicited similar magnitude of changes in alcohol drinking, fear overgeneralization, and irritability-like behavior as seen in our prior work [18]. Table 1 summarizes behavioral effects produced by FKBP5 inhibitors in stressed males and females. In Study 1, benztropine decreased alcohol preference in stressed males and females, while decreasing aggressive-like behavior in stressed females. Benztropine also reduced startle responses to acoustic stimuli in stressed males and females. Interestingly, benztropine only reduced circulating corticosterone in stressed males but not females. We also observed opposing effects of stress response following benztropine administration in unstressed drinking males via increased anxiety-like behavior on the fear overgeneralization test, suggesting off-target effects or anxiogenic-like effects of benztropine under previously unstressed conditions. In Study 2, the selective FKBP5 inhibitor, SAFit2, reduced alcohol drinking in stressed males but not females. Importantly, SAFit2 reduced fear overgeneralization in both stressed males and females as well as corticosterone levels. Neither FKBP5 inhibitor altered sleep bout structure across males or females. Taken together, these findings demonstrate that FKBP5 inhibitors, with some sexually dimorphic action, show promising therapeutic efficacy to reduce some aspects of trauma-related behaviors and alcohol drinking in a model of comorbid PTSD/AUD.
FKBP5 has gained significant interest for its putative involvement in modulating alcohol drinking in humans [11, 13] and rodents [13]. Thus, we hypothesized that administration of FKBP5 inhibitors, benztropine or SAFit2, would normalize alcohol drinking in stressed animals. Here, we demonstrated that the FKBP5 inhibitors decrease trauma-related alcohol drinking in stressed males and females. The reductions in alcohol drinking appear to be selective to animals exposed to traumatic stress history since we observed no drug effects on drinking in unstressed rats following FKBP5 inhibitors. Similar lines of evidence with FKBP5 inhibitors have shown that SAFit2 administration reduces alcohol consumption using 2BC procedures in male mice [31]. The same report also found that selective inhibition of FKBP5 decreased relapse-induced drinking [31]. Other reports suggest opposing effects of FKBP5 in knock-out mice, which exhibited increases in alcohol consumption across a range of alcohol concentrations [35] or a lack of effects produced by FKBP5 inhibition in selectively-bred high drinking mice [36]. The latter finding might suggest an inverse relationship in the role of FKBP5 in stressed versus unstressed conditions to promote alcohol drinking, increased sensitivity to FKBP5 inhibitors after stress history, or, alternatively, opposing effects resulting from constitutive loss versus acute pharmacological reduction of FKBP5 function.
Since FKBP5 is thought to have a strong pathophysiological role in PTSD [10, 14, 37,38,39], we administered the FKBP5 inhibitors, benztropine or SAFit2 and also assessed PTSD-like behaviors during abstinence from alcohol. We hypothesized that benztropine and SAFit2 would also reduce various trauma-related behaviors involved in PTSD, including sleep bout cycles, hyperarousal, fear overgeneralization, and irritability-like behavior [40,41,42]. Furthermore, we found that FKBP5 inhibitors reduced some aspects of PTSD-related behaviors such as hyperarousal and irritability-like behaviors (females only), with stronger effects exerted by benztropine. We also found that SAFit2 reduced fear overgeneralization in stressed males and females. Thus, targeting FKBP5 might help improve some trauma-related symptoms of PTSD which has received increasing interest due to its role in PTSD. A preclinical study using FKBP5 KO mice showed greater resiliency to the effects of social defeat stress and improved stress coping behaviors using forced swim test paradigms [43]. Moreover, co-administration of SAFit2 and escitalopram (i.e., selective serotonin reuptake inhibitor) increased mobility time on the forced swim test apparatus, an indication of improvement of stress coping behavior produced by these drugs [29].
We also demonstrated that FKBP5 inhibitors have some sex-specific effects on trauma-related behaviors and alcohol drinking. The reduction in alcohol drinking following SAFit2 administration were only observed in stressed males but not females. The reductions in aggressive bouts following benztropine were only observed in females. We also consider that trauma-induced increase in drinking were predominantly observed in the males but not females. This pattern of results may be explained by higher basal vulnerability rates of drinking in females in the model where females generally display enhanced drinking patterns compared to males [18]. Our results might suggest that some aspects of trauma and alcohol drinking may be differentially driven by FKBP5 mechanisms in males and females. There is also evidence that FKBP5 interacts not only with glucocorticoid receptors, but also with other steroid receptors, including progesterone, estrogen, and androgen. FKBP5 may inhibit progesterone receptor activity [44], while promoting estrogen and androgen activity [45, 46]. Indeed, gonadal hormones may relate to these differences, as a rodent study demonstrated that FKBP5 inhibition decreased stress-induced drug reinstatement at a timepoint when estrogen was low in females (during metestrus/diestrus) [47]. Future studies are needed to assess the interaction between estrous cyclicity/gonadal hormones on the sensitivity to FKBP5 inhibitors that may influence their behavioral effects on comorbid PTSD/AUD.
The rationale to consider potential sex differences in FKBP5 inhibition for normalizing PTSD responses and reducing alcohol drinking is based on a strong human literature on sex differences regarding disrupted physiological activity and in pharmacotherapy responses for PTSD/AUD. For example, alcohol-induced disruptions in biophysiological markers of adaptive stress response are more common in women than men [48]. The nature and extent of many alterations are also sex-specific (e.g., blunted physiological responses to stress cues, alcohol cues, and alcohol exposure; sensitized emotional response to stress; alterations in hormonal fluctuations) [49]. Furthermore, pharmacotherapy with naltrexone for AUD found that women exhibit poorer treatment response and experience a higher rate and mean number of adverse effects than men, yet while experiencing a significantly greater reduction in craving scores than men [50, 51]. Similarly, PTSD pharmacotherapies with serotonin reuptake inhibitors and several other medication types appear more effective in men than women, a sex difference that remains controversial despite clear differences in medication metabolism and pharmacodynamics [52, 53].
While broadly similar in reducing drinking and PTSD-like behaviors, benztropine and SAFit2 produced non-overlapping reductions on some behavioral assays (i.e., 2BC alcohol drinking, hyperarousal, and irritability-like behavior). This discrepancy might be explained by pharmacokinetic differences and pharmacological actions of these compounds among males and females. For instance, it is known that benztropine has non-FKBP5 actions and it is classically considered a blocker of cholinergic and histaminergic signaling as well as dopamine transport [54]. However, the functional relevance of its inhibition of FKBP51 remains important since benztropine has shown to increase GR activity in numerous human cell types, increase GR nuclear translocation in mouse neuronal culture through FKBP5 inhibition, and contains significant selectivity for FKBP51 over its functional opposing homolog, FKBP52 [25, 55]. Furthermore, SAFit2 modulates other non-GR actions of FKBP5 suggesting modulation of PTSD/AUD via non-direct GR mechanisms [56, 57]. Thus, this novel mechanism of inhibiting FKBP5 suggests that FKBP5 inhibitors could potentially alleviate PTSD and AUD comorbidity. In support of this, benztropine analogs can reduce cocaine self-administration in rats [58], and during cocaine withdrawal Fkbp5 mRNA is upregulated in brain regions associated with drug use [59]. Therefore, these studies support the notion that inhibiting FKBP5 may be effective in reducing PTSD and AUD comorbidity, distinctly across males and females, and some trauma-related symptoms.
There are some potential mechanistic links to consider based on prior work showing increased CeA GABAergic transmission after stress and alcohol drinking history [18, 23]. Here, we speculate that familiar shock stress and alcohol history synergistically may affect/alter CeA GABAergic signaling, possibly through FKBP5/GR mechanisms (e.g., facilitating ligand activation of GR and its subsequent translocation into the nucleus) [14]. This notion is also supported by other reports indicating that GR antagonists prevent and reverse increased alcohol self-administration associated with alcohol dependence [60,61,62,63,64] and genetically-selected, alcohol preferring models [36], reduced CeA GABA transmission in alcohol dependent rats [65], as well as Fkbp5 gene expression [66]. Future work is needed to identify the specific brain regions involved, and assess the interaction between GR translocation, feedback and expression following treatment with FKBP5 inhibitors in PTSD/AUD comorbidity. We also cannot exclude that other non-GR mediated functions of FKBP5 may contribute to observed effects, including interactions with cholinergic and histaminergic systems [54] as well as serine/threonine protein kinase AKT sites [56, 57].
Collectively, our results indicate that FKBP5 inhibitors reduced some trauma-related behaviors and alcohol drinking in a model of comorbid PTSD/AUD. Our work has strong translational value that suggests the repurposing of benztropine (Cogentin®) or investigation of SAFit2 may be beneficial in suppressing comorbid PTSD/AUD. Since there are no current FDA-approved treatments to alleviate comorbid PTSD/AUD [67], the applicability of this work might suggest that approaches to target shared molecular systems such as FKBP5 is one strategy to ameliorate these co-occurring disorders. Our work also suggests that males and females demonstrate unique biological differences that imply distinct responding to treatment with FKBP5 inhibitors, suggesting that treatment for comorbid PTSD/AUD might require a tailored approach in males and females. Lastly, future studies are required to provide a deeper understanding of the molecular and synaptic mechanisms of FKBP5 and its inhibitors.
References
Blanco C, Xu Y, Brady K, Perez-Fuentes G, Okuda M, Wang S. Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: results from National Epidemiological Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2013;132:630–8.
Debell F, Fear NT, Head M, Batt-Rawden S, Greenberg N, Wessely S, et al. A systematic review of the comorbidity between PTSD and alcohol misuse. Soc Psychiatry Psychiatr Epidemiol. 2014;49:1401–25.
Shorter D, Hsieh J, Kosten TR. Pharmacologic management of comorbid post-traumatic stress disorder and addictions. Am J Addict. 2015;24:705–12.
Evren C, Dalbudak E, Evren B, Cetin R, Durkaya M. Self-mutilative behaviours in male alcohol-dependent inpatients and relationship with posttraumatic stress disorder. Psychiatry Res. 2011;186:91–6.
Rojas SM, Bujarski S, Babson KA, Dutton CE, Feldner MT. Understanding PTSD comorbidity and suicidal behavior: associations among histories of alcohol dependence, major depressive disorder, and suicidal ideation and attempts. J Anxiety Disord. 2014;28:318–25.
Hoerster KD, Malte CA, Imel ZE, Ahmad Z, Hunt SC, Jakupcak M. Association of perceived barriers with prospective use of VA mental health care among Iraq and Afghanistan veterans. Psychiatr Serv. 2012;63:380–2.
Norman SB, Haller M, Hamblen JL, Southwick SM, Pietrzak RH. The burden of co-occurring alcohol use disorder and PTSD in U.S. Military veterans: comorbidities, functioning, and suicidality. Psychol Addict Behav. 2018;32:224–29.
Zoricic Z, Karlovic D, Buljan D, Marusic S. Comorbid alcohol addiction increases aggression level in soldiers with combat-related post-traumatic stress disorder. Nord J Psychiatry. 2003;57:199–202.
Kang JI, Kim, TY, Chung, HG, Choi, JH, Hwang, EH, Kim, SJ. Genetic and epigenetic involvement of the FKBP5 gene in posttraumatic stress disorder after combat trauma. Eur Neuropsychopharmacol. 2019;29:S853–54.
Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, et al. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–92.
Dragan WL, Domozych W, Czerski PM, Dragan M. Positive metacognitions about alcohol mediate the relationship between FKBP5 variability and problematic drinking in a sample of young women. Neuropsychiatr Dis Treat. 2018;14:2681–88.
Huang MC, Schwandt ML, Chester JA, Kirchhoff AM, Kao CF, Liang T, et al. FKBP5 moderates alcohol withdrawal severity: human genetic association and functional validation in knockout mice. Neuropsychopharmacology. 2014;39:2029–38.
Nylander I, Todkar A, Granholm L, Vrettou M, Bendre M, Boon W, et al. Evidence for a link between Fkbp5/FKBP5, early life social relations and alcohol drinking in young adult rats and humans. Mol Neurobiol. 2017;54:6225–34.
Zannas AS, Wiechmann T, Gassen NC, Binder EB. Gene-stress-epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacology. 2016;41:261–74.
Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–16.
Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34:S186–95.
Scharf SH, Liebl C, Binder EB, Schmidt MV, Muller MB. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS One. 2011;6:e16883.
Steinman MQ, Kirson D, Wolfe SA, Khom S, D’Ambrosio SR, Spierling Bagsic SR, et al. Importance of sex and trauma context on circulating cytokines and amygdalar GABAergic signaling in a comorbid model of posttraumatic stress and alcohol use disorders. Mol Psychiatry. 2021;26:3093–107.
Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl). 2011;218:131–56.
Daskalakis NP, Yehuda R, Diamond DM. Animal models in translational studies of PTSD. Psychoneuroendocrinology. 2013;38:1895–911.
Natividad LA, Steinman MQ, McGinn MA, Sureshchandra S, Kerr TM, Ciccocioppo R, et al. Impaired hypothalamic feedback dysregulates brain glucocorticoid signaling in genetically-selected Marchigian Sardinian alcohol-preferring rats. Addict Biol. 2021;26:e12978.
Natividad LA, Buczynski MW, Herman MA, Kirson D, Oleata CS, Irimia C, et al. Constitutive increases in amygdalar corticotropin-releasing factor and fatty acid amide hydrolase drive an anxious phenotype. Biol Psychiatry. 2017;82:500–10.
Kirson D, Steinman MQ, Wolfe SA, Spierling Bagsic SR, Bajo M, Sureshchandra S, et al. Sex and context differences in the effects of trauma on comorbid alcohol use and post-traumatic stress phenotypes in actively drinking rats. J Neurosci Res. 2021;99:3354–72.
Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol Rev. 2001;108:4–32.
Sabbagh JJ, Cordova RA, Zheng D, Criado-Marrero M, Lemus A, Li P, et al. Targeting the FKBP51/GR/Hsp90 complex to identify functionally relevant treatments for depression and PTSD. ACS Chem Biol. 2018;13:2288–99.
Gaali S, Kirschner A, Cuboni S, Hartmann J, Kozany C, Balsevich G, et al. Selective inhibitors of the FK506-binding protein 51 by induced fit. Nat Chem Biol. 2015;11:33–7.
Jones CK, Shannon HE. Muscarinic cholinergic modulation of prepulse inhibition of the acoustic startle reflex. J Pharm Exp Ther. 2000;294:1017–23.
Hartmann J, Wagner KV, Gaali S, Kirschner A, Kozany C, Ruhter G, et al. Pharmacological inhibition of the psychiatric risk factor FKBP51 has anxiolytic properties. J Neurosci. 2015;35:9007–16.
Pohlmann ML, Hausl AS, Harbich D, Balsevich G, Engelhardt C, Feng X, et al. Pharmacological modulation of the psychiatric risk factor FKBP51 alters efficiency of common antidepressant drugs. Front Behav Neurosci. 2018;12:262.
Müller CKL, Kalinichenko L, Huber S, Voll A, Bauder M, Kornhuber J, et al. Pharmacological inhibition of FK506-binding protein 51 reduces alcohol consumption and conditioned place preference in mice. Pharmacopsychiatry. 2019;52:95.
Konig L, Kalinichenko LS, Huber SE, Voll AM, Bauder M, Kornhuber J, et al. The selective FKBP51 inhibitor SAFit2 reduces alcohol consumption and reinstatement of conditioned alcohol effects in mice. Addict Biol. 2020;25:e12758.
Maiaru M, Morgan OB, Mao T, Breitsamer M, Bamber H, Pohlmann M, et al. The stress regulator FKBP51: a novel and promising druggable target for the treatment of persistent pain states across sexes. Pain. 2018;159:1224–34.
Carey RJ. A comparison of atropine, benztropine and diphenhydramine on the reversal of haloperidol induced suppression of self-stimulation. Pharm Biochem Behav. 1982;17:851–4.
Vozella V, Cruz B, Natividad LA, Benvenuti F, Cannella N, Edwards S, et al. Glucocorticoid receptor antagonist mifepristone does not alter innate anxiety-like behavior in genetically-selected marchigian sardinian (msP) rats. Int J Mol Sci. 2021;22:3095.
Qiu B, Luczak SE, Wall TL, Kirchhoff AM, Xu Y, Eng MY, et al. The FKBP5 gene affects alcohol drinking in knockout mice and is implicated in alcohol drinking in humans. Int J Mol Sci. 2016;17:1271.
Savarese AM, Ozburn AR, Metten P, Schlumbohm JP, Hack WR, LeMoine K, et al. Targeting the glucocorticoid receptor reduces binge-like drinking in high drinking in the dark (HDID-1) mice. Alcohol Clin Exp Res. 2020;44:1025–36.
Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–305.
Watkins LE, Han S, Harpaz-Rotem I, Mota NP, Southwick SM, Krystal JH, et al. FKBP5 polymorphisms, childhood abuse, and PTSD symptoms: results from the National Health and Resilience in Veterans Study. Psychoneuroendocrinology. 2016;69:98–105.
Sinclair D, Fillman SG, Webster MJ, Weickert CS. Dysregulation of glucocorticoid receptor co-factors FKBP5, BAG1 and PTGES3 in prefrontal cortex in psychotic illness. Sci Rep. 2013;3:3539.
Kivisto AJ, Moore TM, Elkins SR, Rhatigan DL. The effects of PTSD symptomatology on laboratory-based aggression. J Trauma Stress. 2009;22:344–7.
Babcock JC, Roseman A, Green CE, Ross JM. Intimate partner abuse and PTSD symptomatology: examining mediators and moderators of the abuse-trauma link. J Fam Psychol. 2008;22:809–18.
Williamson JB, Jaffee MS, Jorge RE. Posttraumatic stress disorder and anxiety-related conditions. Continuum (Minneap Minn). 2021;27:1738–63.
Hartmann J, Wagner KV, Liebl C, Scharf SH, Wang XD, Wolf M, et al. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology. 2012;62:332–9.
Barent RL, Nair SC, Carr DC, Ruan Y, Rimerman RA, Fulton J, et al. Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol Endocrinol. 1998;12:342–54.
Shrestha S, Sun Y, Lufkin T, Kraus P, Or Y, Garcia YA, et al. Tetratricopeptide repeat domain 9A negatively regulates estrogen receptor alpha activity. Int J Biol Sci. 2015;11:434–47.
Stechschulte LA, Sanchez ER. FKBP51-a selective modulator of glucocorticoid and androgen sensitivity. Curr Opin Pharm. 2011;11:332–7.
Connelly KL, Wolsh CC, Barr JL, Bauder M, Hausch F, Unterwald EM. Sex differences in the effect of the FKBP5 inhibitor SAFit2 on anxiety and stress-induced reinstatement following cocaine self-administration. Neurobiol Stress. 2020;13:100232.
Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, et al. Sex differences in stress-related alcohol use. Neurobiol Stress. 2019;10:100149.
Fox HC, Sinha R. Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women. Harv Rev Psychiatry. 2009;17:103–19.
Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–25.
Herbeck DM, Jeter KE, Cousins SJ, Abdelmaksoud R, Crevecoeur-MacPhail D. Gender differences in treatment and clinical characteristics among patients receiving extended release naltrexone. J Addict Dis. 2016;35:305–14.
Huang ZD, Zhao YF, Li S, Gu HY, Lin LL, Yang ZY, et al. Comparative efficacy and acceptability of pharmaceutical management for adults with post-traumatic stress disorder: a systematic review and meta-analysis. Front Pharm. 2020;11:559.
Sramek JJ, Murphy MF, Cutler NR. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin Neurosci. 2016;18:447–57.
Rothman RB, Baumann MH, Prisinzano TE, Newman AH. Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem Pharm. 2008;75:2–16.
Sabban EL, Serova LI, Newman E, Aisenberg N, Akirav I. Changes in gene expression in the locus coeruleus-amygdala circuitry in inhibitory avoidance PTSD model. Cell Mol Neurobiol. 2018;38:273–80.
Feng X, Pomplun S, Hausch F. Recent progress in FKBP ligand development. Curr Mol Pharm. 2015;9:27–36.
Balsevich G, Hausl AS, Meyer CW, Karamihalev S, Feng X, Pohlmann ML, et al. Stress-responsive FKBP51 regulates AKT2-AS160 signaling and metabolic function. Nat Commun. 2017;8:1725.
Zanettini C, Wilkinson DS, Katz JL. Behavioral economic analysis of the effects of N-substituted benztropine analogs on cocaine self-administration in rats. Psychopharmacology (Berl). 2018;235:47–58.
Connelly KL, Unterwald EM. Chronic cocaine administration upregulates FKBP5 in the extended amygdala of male and female rats. Drug Alcohol Depend. 2019;199:101–05.
Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW Jr, Logrip ML, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–71.
Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, et al. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest. 2015;125:3193–7.
Benvenuti F, Cannella N, Stopponi S, Soverchia L, Ubaldi M, Lunerti V, et al. Effect of glucocorticoid receptor antagonism on alcohol self-administration in genetically-selected marchigian sardinian alcohol-preferring and non-preferring wistar rats. Int J Mol Sci. 2021;22:4184.
Somkuwar SS, Vendruscolo LF, Fannon MJ, Schmeichel BE, Nguyen TB, Guevara J, et al. Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology. 2017;84:17–31.
McGinn MA, Tunstall BJ, Schlosburg JE, Gregory-Flores A, George O, de Guglielmo G, et al. Glucocorticoid receptor modulators decrease alcohol self-administration in male rats. Neuropharmacology. 2021;188:108510.
Khom S, Rodriguez L, Gandhi P, Kirson D, Bajo M, Oleata CS, et al. Alcohol dependence and withdrawal increase sensitivity of central amygdalar GABAergic synapses to the glucocorticoid receptor antagonist mifepristone in male rats. Neurobiol Dis. 2022;164:105610.
Bali U, Phillips T, Hunt H, Unitt J. FKBP5 mRNA expression is a biomarker for GR antagonism. J Clin Endocrinol Metab. 2016;101:4305–12.
Ralevski E, Olivera-Figueroa LA, Petrakis I. PTSD and comorbid AUD: a review of pharmacological and alternative treatment options. Subst Abus Rehabil. 2014;5:25–36.
Acknowledgements
The authors thank Dr. Luisa B. Bertotto for her technical support with experiments and Shannon R. D’Ambrosio for assisting during shock stress procedures.
Funding
Support for this study was provided by The National Institute on Alcohol Abuse and Alcoholism grants, AA027700, AA028879, AA013498, P60 AA006420, AA017447, AA021491, AA029841, AA015566, K99 AA026638, T32 AA007456, and the Schimmel Family Endowed Chair. The Department of Defense (DoD) provided support for the Pharmacotherapies for Alcohol and Substance use disorders Alliance (PASA). The Alcohol and Substance Use Disorders Research Program (ASUDRP) Programmatic Panel is a Department of Defense (DoD) appointed and chaired steering committee that provides oversight to the PASA Consortium. The ASUDRP Programmatic Panel is chaired by DoD and comprises government representatives and non-DoD subject matter experts. The panel approves all studies to be conducted, recommends new studies, and identifies existing and new requirements as they arise. The ASUDRP Programmatic Panel is the overall main governing and management committee and the committee through which the DoD interacts and collaborates with the PASA Consortium. The ASUDRP Programmatic Panel determines all major scientific decisions; clinical studies proposed by the Consortium Committee proceed into the implementation stage only with the approval of the ASUDRP Programmatic Panel. While the DoD/ASADRP had input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of DoD, ASUDRP, or the U.S. Government. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Alcohol and Substance Use Research Program under Award No. W81XWH1820044. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. In conducting research using animals, the investigator(s) adhered to the laws of the United States and regulations of the Department of Agriculture. Data were collected at participating sites of the PASA consortium and were transmitted to RTI International for this study. RTI International has full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. This is manuscript number 30175 from The Scripps Research Institute.
Author information
Authors and Affiliations
Contributions
BC, VV, EPZ, and MR designed and conceived the project. BC and VV collected behavioral and blood plasma data. BC, BAC, and SH analyzed behavioral data. BC and VV analyzed the blood plasma data. BC, VV, EPZ, and MR drafted the figures and manuscript. BC, VV, BAC, JCX, DK, SH, TN, LB, KF, MC, TRK, EPZ, and MR commented and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Cruz, B., Vozella, V., Carper, B.A. et al. FKBP5 inhibitors modulate alcohol drinking and trauma-related behaviors in a model of comorbid post-traumatic stress and alcohol use disorder. Neuropsychopharmacol. 48, 1144–1154 (2023). https://doi.org/10.1038/s41386-022-01497-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01497-w
This article is cited by
-
A systematic review and meta-analysis on the transcriptomic signatures in alcohol use disorder
Molecular Psychiatry (2025)
-
Childhood trauma cortisol and immune cell glucocorticoid transcript levels are associated with increased risk for suicidality in adolescence
Molecular Psychiatry (2025)
-
Chemogenetic inhibition of central amygdala CRF-expressing neurons decreases alcohol intake but not trauma-related behaviors in a rat model of post-traumatic stress and alcohol use disorder
Molecular Psychiatry (2024)
-
Alcohol-specific transcriptional dynamics of memory reconsolidation and relapse
Translational Psychiatry (2023)
-
Single-nucleus genomics in outbred rats with divergent cocaine addiction-like behaviors reveals changes in amygdala GABAergic inhibition
Nature Neuroscience (2023)